Thursday, July 9, 2009
Lab 2: IR Identification of Analgesics By: Alice Story
Introduction: Infared Spectroscopy is a forensic technique used to analyze organic compounds . Infared radiation identifies compounds when infared light strikes an object. The spectrum starts where the red region of visible light ends.
Materials and Methods: An unknown saturated solution of samples 2 and 7 were prepared. On one side of the IR cards one sample was placed on to the card. The card was placed into the spectroscopy on the side that did not contain any sample (background). Then the card was placed on the side containg sample 2 (repeated for sample 7) and the spectroscopy created a graph to later determine the functional groups.
Results: The readings from the spectroscopy graph identified the unknown samples 2 and 7. Sample 2 contained Aspirin and Tylenol PM. Sample 7 was Aspirin.
Discussion: The spectroscopy identified the unknown samples 2 and 7 by identifying specific functional groups. The determination of the specific functional groups determine the substance(s) in each sample.
Lab 2: IR Identification of Analgesics by Brittani Summers
Introduction: Infrared Spectrometers use radiation to identify certain substances. The radiation from the light strikes the substance and the spectrometer shows waves. The peaks from the waves show the different types of chemical bonds formed in the substance.
Materials:
- IR Cards
- IR Spectrometer
- Samples 2 & 5
Procedure:
- Side A of one IR card was placed into the Spectrometer and served as the background/control of the experiment.
- Side B of one of the IR Cards was placed into the Spectrometer containing Sample 2.
- Side B of the other IR Card was placed into the Spectrometer containing Sample 5.
- The graph of the the transmittance of the two substances was then printed.
- Later on, the drugs within the substance was then discovered by the peaks of the waves transmitted through the Spectrometer. The use of table titled "Characteristic Infrared Absorption Frequencies" was used to discover the bonds featured in the structures of the possible drugs in the mixture.
Results/Discussion/Conclusion:
Sample 2 contained each of the following:
1750 absorbance: C=O ester
1300 absorbance: C--O alcohols, ethers, and esters
2900 absorbance: O--H acids
Sample 2 contained Aspirin as well as Tylenol
Sample 5 contained each of the following:
3300 absorbance: N--H amines
2900 absorbance: O--H acids
1300 absorbance: C--O alcohols, ethers, esters
Sample 5 contained Tylenol and Ibuprofen
Lab 2: IR Identification of Analgesics by Carla Scott
Introduction:
Infrared Spectroscopy is a tool commonly used in identifying chemical compounds by. This technique is used most often in forensic laboratories to determine whether analgesic or prescription drugs were used in criminal investigations.
Materials: Saturated solutions, IR Cards, and Spectroscometer
Procedures:
Saturated solutions 1 drop of sample 4 & 6 was placed on the film of IR cards and placed in the Spectrometer and read.
Results/Data: Based on the readings from the Infrared Spectroscometer the following findings were found:
Sample 4 was determined to be Ibuprofen
Sample 6 was determined to be Tylenol
Discusssion & Conclusion:
This laboratory experiment's goal was to use Infrared Spectroscopy to determine which analgesics were present. I found that my samples contained Ibuprofen (sample 4) and Tylenol (sample 6) which are both over-the-counter drugs.
By Carla Scott
Lab 2: IR Identification of Analgesics by Serina Lewis
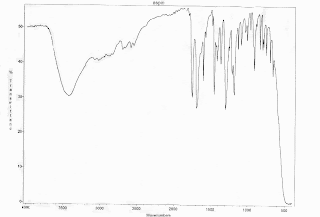
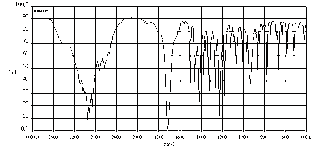
-Serina Lewis
Lab 1: Identification of Analgesics using Thin Layer Chromatography by Carla Scott

Lab 1: Identification of Analgesics using TLC by Alice Story
Introduction: Drug identification is determined by TLC, which is a analytical technique used to analyze the purity of chemical compounds.

Figure 1. TLC Samples 2 and 7
Discussion: This experiment allowed the determination of purity of samples 2 and 7. The results determined that sample 2 spotting resulted in a streak. Sample 2 streaking was due to an overload or many components ran together creating a streak. Sample 7 resulted in a pure substance since there was no abnormality to the spot.
Lab 1: Identification of Analgesics by Kaneshia Newell
 nd mixed it with ethyl acetate and acetic acid. We drew a line 1 cm from the bottom of the verticle TLC plate with a pencil. Using a capillary tube, placed a few spots on the the TLC plate. Place a filter paper in a 400 mL beaker with the solvent. Once the TLC plate dries, placed it in the solvent of ethanol and acetic acid until the product diffuses 1 inch from the top. Observe data and record results using the UV lamp for detailed observations. To make results more visible, we used iodide in a beaker as seen in Figure 1.1.
nd mixed it with ethyl acetate and acetic acid. We drew a line 1 cm from the bottom of the verticle TLC plate with a pencil. Using a capillary tube, placed a few spots on the the TLC plate. Place a filter paper in a 400 mL beaker with the solvent. Once the TLC plate dries, placed it in the solvent of ethanol and acetic acid until the product diffuses 1 inch from the top. Observe data and record results using the UV lamp for detailed observations. To make results more visible, we used iodide in a beaker as seen in Figure 1.1.by: Kanieshia N.
Lab 1: Identification of Analgesics by Serina Lewis
Lab 1 Identification of Analgesics Using TLC by: Brittani Summers
- Unknown Sample 2 (1 % solution) prepared in ethanol, ethyl acetate and acetic acid
- Unknown Sample 5 (1 % solution) prepared in ethanol, ethyl acetate and acetic acid
- Iodine
- UV lamp
- TLC Plates
- Beaker with watch glass
- Ruler
- Calculator
Procedure:
- A line was drawn 1 centimeter from the bottome of the TLC plate
- A capillary tube was used to spot the TLC plate at the 1 centimeter line.
- The TLC plate was then placed inside the beaker containing ethyl acetate and acetic acid and covered with a watch glass.
- The mixture of ethyl acetate and acetic acid then began to absorb through the TLC plate. The TLC plate were then removed once the solvent reached approximately 1 inch from the top of the TLC plate.
- The wet TLC plates were then left to dry.
- The dried TLC plates were then observed under the UV lamp.
- The plates were then placed in a beaker with solid iodine and the vapors from the iodine left more distinct marks on the TLC plates.
- The TLC plates were then viewed again under the UV lamp and marks were made where the solution stopped flowing on the TLC plate.
- With the ruler, measurements of where the solvent stopped and where the substance stopped were taken note of in centimeters.
- The Rf values were then calculated by dividing the distance the substance traveled by the distance the solvent traveled.
- Rf= distance substance traveled/ distance solvent traveled
Results/Discussion/Conclusion:
Substance 2 had a Rf value of .613 Figures 1: Samples 2 & 5
Figures 1: Samples 2 & 5
Substance 5 had a Rf value of .565
The TLC plates showed several different spots in one line. Because there were several different spots in one line, it comes to a conclusion that the substance is not pure. Both substances 2 and 5 revealed several different spots so the conclusion was made that these two substances were not pure, therefore being mixed with something else.
Figure 2: Samples 2 & 5


