Introduction: The Fourier Transform Infrared Spectrophotometer is used by forensic labs to identify drugs and organic poisons in powders, urine and tablets. The instrument measures the frequencies at which a group of atoms in a sample rotates and vibrates in a molecule. IR spectroscopy, most often used in criminalistics, is used to determine what is present. Two regions of interest when measuring the absorption of infrared radiation are the functional group region and the fingerprint region. The functional group region shows the absorption patterns for each of the functional groups present. The fingerprint region shows a pattern of each molecule's absorption bands and is formed when the atoms in the molecule are vibrating. These regions contribute to the difficult mathematical procedure used to get the data that forms the IR spectroscopy chart.
Materials: Samples 4 and 7 (1% saturated solution), pipette, IR cards, and IR spectrophotometer
Procedures: A drop from a pipette of each sample were placed on separate IR cards. Then, the IR cards were left to dry. Once the sample on the IR card dried, the IR card was placed into the spectrophotometer and the spectrum was obtained.
Results
Chart 1, below, sample IR spectra for sample 4
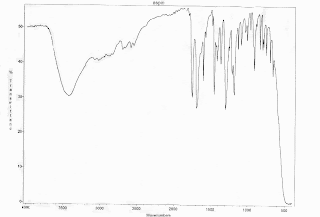
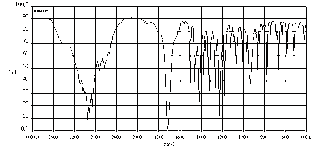
Chart 2, below, sample IR spectra for sample 7
Sample 4- absorption of 3000-2500 cm
Sample 7-absorption of 1725-1705 cm
Discussion/Conclusion: The IR spectra chart was compared with the characteristic infrared absorption frequencies chart given from the instructor. It was concluded that Sample 4 is ibuprofen and Sample 7 is aspirin.
-Serina Lewis
No comments:
Post a Comment